- Abstract
- Introduction
- CNS tropism of Lm after nasal challenge
- Invasion of the olfactory epithelium
- Lm disrupts the OE and migrates along sensory nerves into the CN
- Accentuated inflammatory response in the OB upon Lm infection
- Lm induces influx of monocytes and neutrophils into the CNS
- Conclusion
- Original Poster
The olfactory epithelium as a portal of entry in neonatal CNS infection
MedUP rookie
D. Pägelow, C. Chhatbar, X. Liu, A. Beineke, M. Rohde, A. Nerlich, K. van Vorst, U. Kalinke, R. Förster, P. Valentin- Weigand, S. Halle, M. Hornef, M. Fulde
Poster presentation on International Symposium on Zoonoses Research 2018 (Poster presentation award: 3rd place)
Introduction
Bacterial infections with a manifestation in the central nervous system (CNS) represent an important cause of morbidity and mortality in neonates. Listeria monocytogenes (Lm), a Gram-positive, facultative intracellular bacterium, is considered to be one of the major neuroinvasive pathogens, with case fatality rates up to 30 % and severe sequelae in survivors. However, mechanisms of host susceptibility, route of infection and underlying mechanisms of inflammation in the CNS remain ill-defined. To our knowledge, up to date there is no small animal model for neonatal neurolisteriosis available. The aim of this study was to establish a robust in vivo model of neonatal CNS infection with Lm following mucosal challenge, in order to investigate the cellular and molecular mechanisms of bacterial tissue tropism as well as innate immune responses.
CNS tropism of Lm after nasal challenge
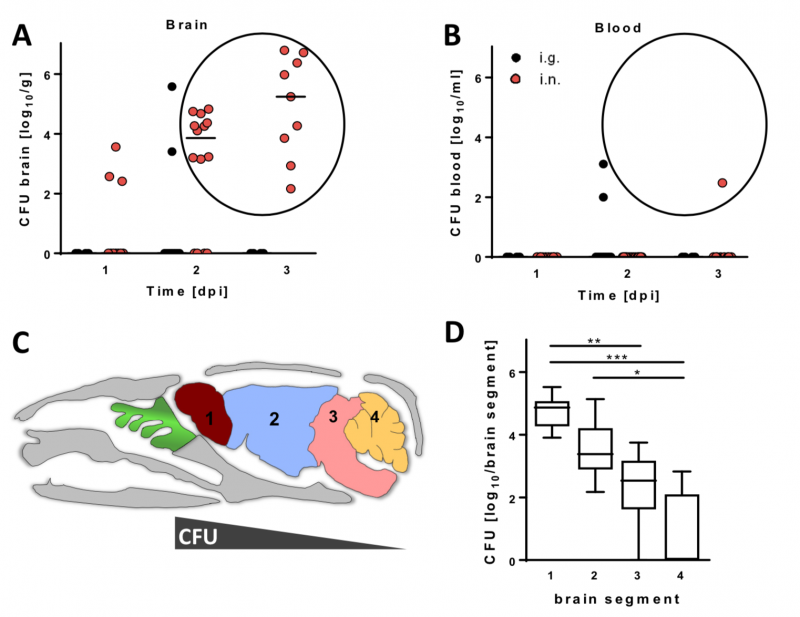
Image 1 Neonatal C57BL/6 mice were infected either intranasally (i.n.) with 4 x 104 or intragastrically (i.g.) with 5 x 107 CFU Lm EGDe. Number of viable bacteria in (A) brain and (B) blood at 1-3 days post infection (dpi). (C) Sagittal illustration of a mouse skull. Mice were i.n. infected as in (A), sacrificed at 3 dpi, perfused and brains dissected into 1) olfactory bulb, 2) cerebrum, 3) brain stem and 4) cerebellum. (D) Number of viable bacteria within the different brain parts.
Invasion of the olfactory epithelium
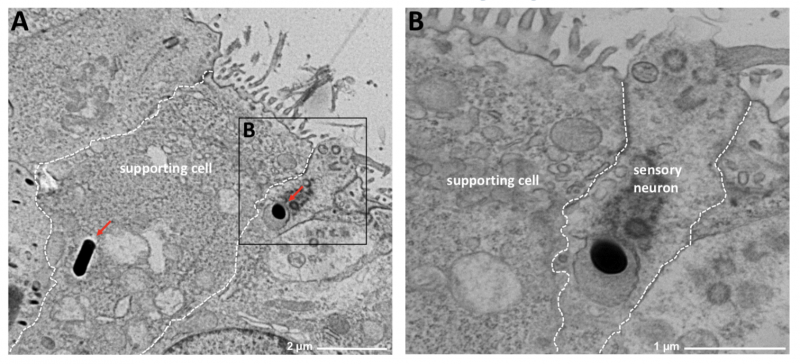
Image 2 Transmission electron microscopy (TEM) of the olfactory epithelium (OE). Invasion of Lm EDGe into nasal mucosae of newborn mice was found exclusively associated to the OE. (A) Intracytosolic Lm within a supporting cell and (B) intravacuolar within an olfactory sensory neuron at 12 hours post infection.
Lm disrupts the OE and migrates along sensory nerves into the CN
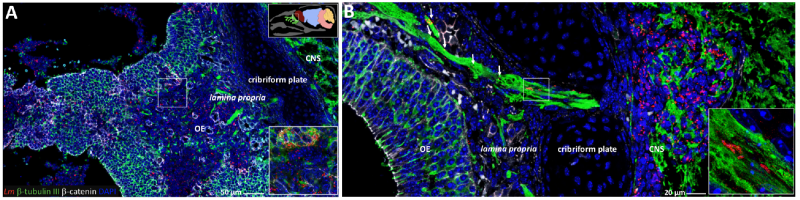
Image 3 Longitudinally bisected head sections were stained for Lm, the neuronal marker β-tubulin, the tight junction marker β-catenin and DNA (DAPI). (A) Lm induced multifocal disruption of the OE integrity. (B) Lm is associated to olfactory axon bundles in the lamina propria and cribriform plate.
Accentuated inflammatory response in the OB upon Lm infection
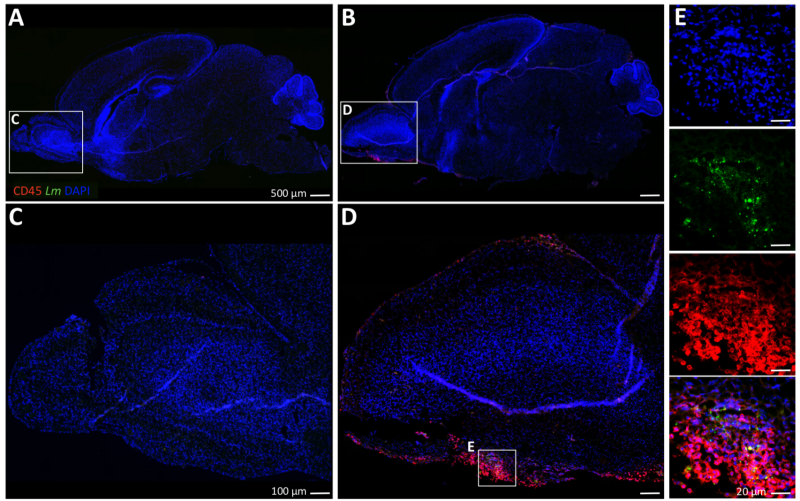
Image 4 Sagittally bisected brains were stained for the pan- leukocyte marker CD45, Lm and DNA (DAPI). (A and C) Overview of a non-infected neonatal C57BL/6 brain. (B and D) I.n. infection with Lm EDGe induces accumulation of a CD45+ cell response accentuated in the olfactory bulb (OB) by 3 dpi. (E) Listeria are found co-localized to CD45+ cells within the OB.
Lm induces influx of monocytes and neutrophils into the CNS
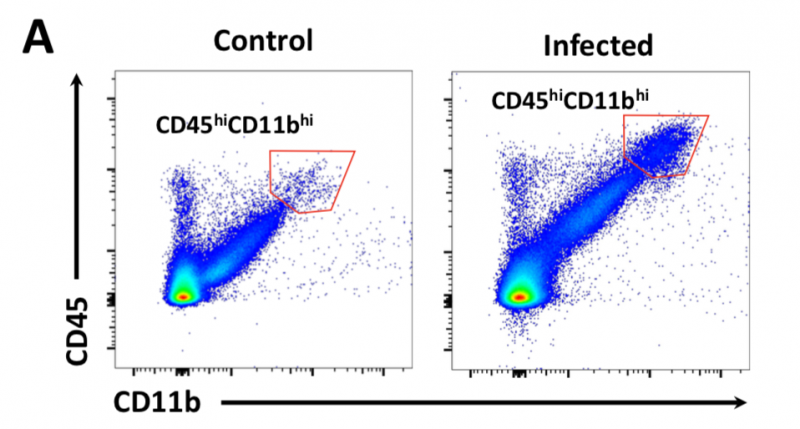
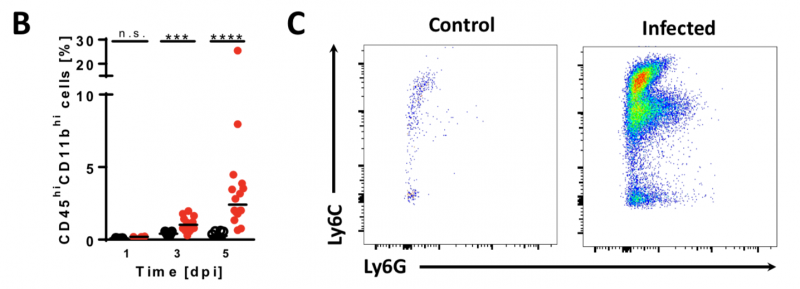
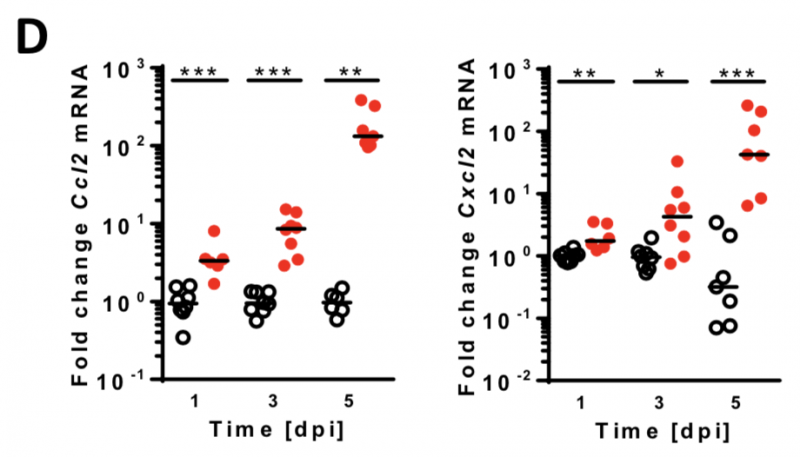
Image 5 A-B Neonatal mice were given PBS (¡) or infected i.n. with Lm (●). (A) Brain cell lysates were analyzed via FACS for CD45 and the pan-myeloid marker CD11b. (B) Significant influx of myeloid immune cells (red pentagon in A). (C) The same cell populations were analyzed for the monocyte marker Ly6C and the neutrophil marker Ly6G. (D) qRT-PCR of monocyte- and neutrophil attracting chemokines Ccl2 and Cxcl2, respectively.
Conclusion
In this study, we present a novel murine infection model for neonatal neurolisteriosis and demonstrate that Lm EGDe is able to translocate through the OE and migrate along olfactory axon bundles into the CNS. Further, immune histological staining, FACS and qRT-PCR revealed potent immune responses in the brain tissue with a primary focus in the olfactory bulbs and frontal lobe. This model allows the dissection of the molecular and cellular processes of Lm mucosal translocation, CNS tropism and local host immune stimulation after challenge via the natural route.
Original Poster